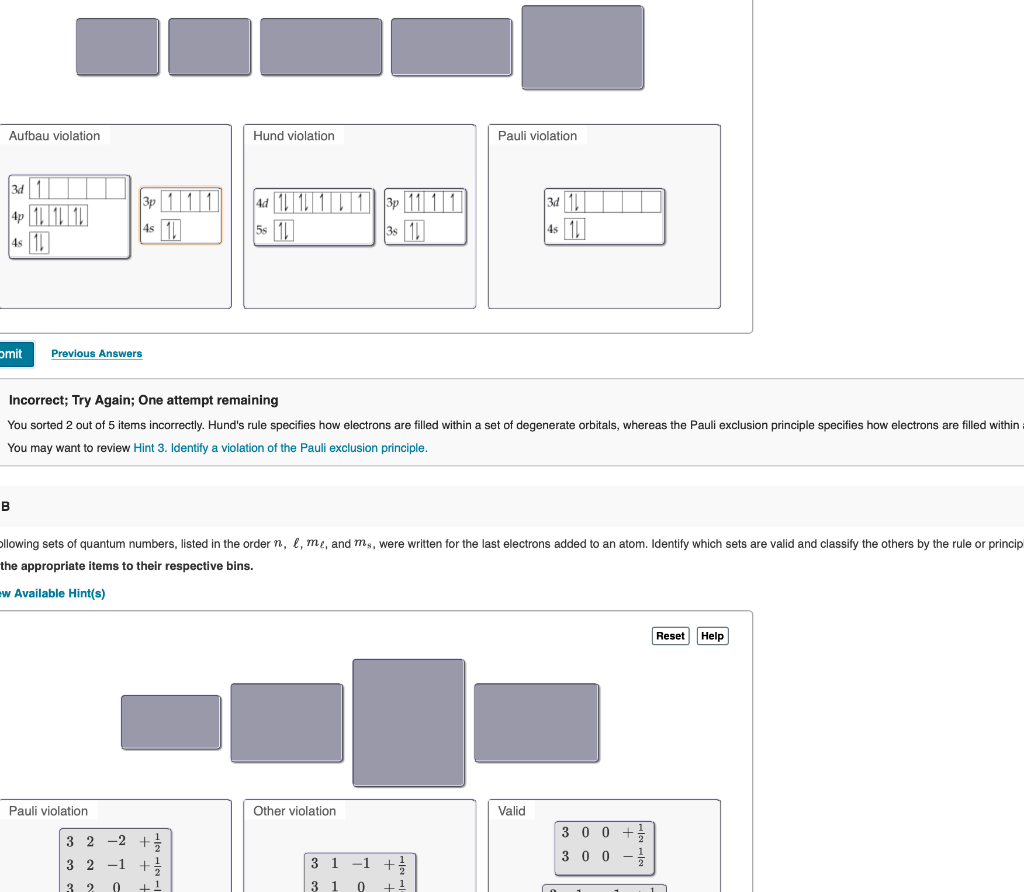
Hund S Rule Violation Example
Sublevels can be broken down into regions called orbitals.
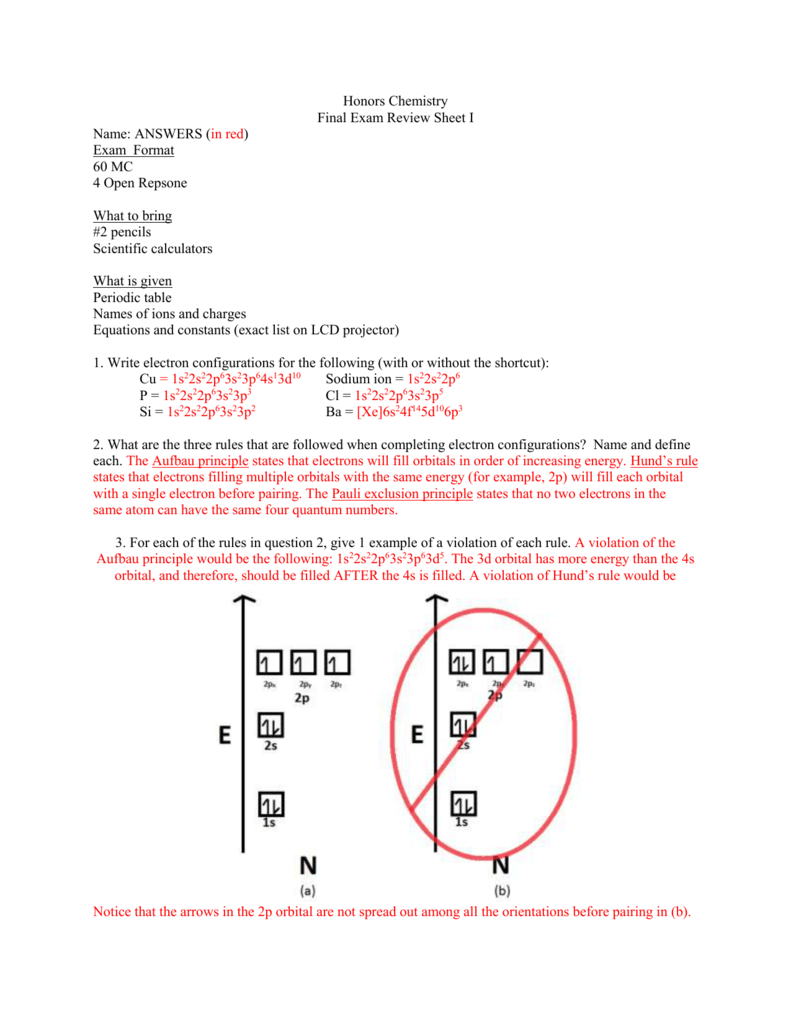
Hund s rule violation example. 1s 2 2s 2 2p 2. The superscript 3 is the value of the multiplicity 2s 1 3.
Pauli exclusion principle and hund s rule. Electrons will be in either positive half spin 1 2 or negative half spin 1 2 for example argon s electron configuration.
Fill from the bott. Hunds rule of maximum multiplicity rule states that for a given electron configuration the term with maximum multiplicity falls lowest in energy.
It is called the box and arrow or circle and x orbital configuration. They are rules we use to fill electron orbital filling diagrams.
Consider the electron configuration for carbon atoms. Consider also the electron configuration of oxygen.
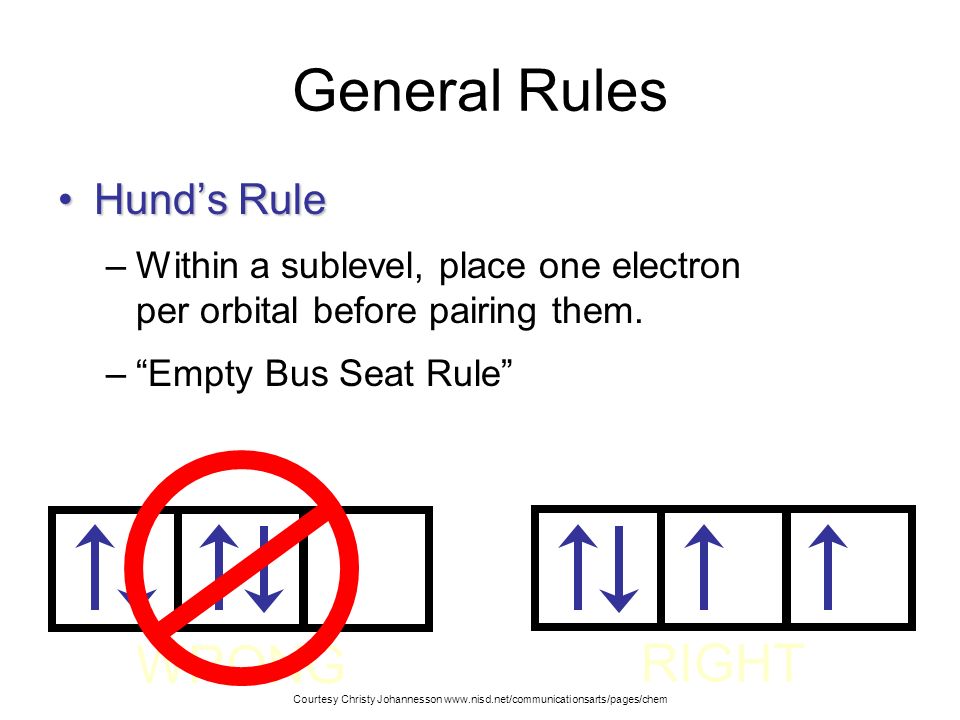
According to this rule electron pairing in p d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied. An orbital is defined as the most probable location for finding an electron.
The two 2s electrons will occupy the same orbital whereas the two 2p electrons will be in different orbital and aligned the same direction in accordance with hund s rule. There is yet another way to writing electron configurations.
Although most stable molecules have closed electron shells a few have unpaired electrons for which hund s rule is applicable. Hund s first rule now states that the ground state term is 3 p which has s 1.
What are the pauli exclusion principle aufbau principle and hunds rule. 1s2 2s2 2p6 3s2 3p6.
The diagram shows the state of this term with m l 1 and m s 1.