Hund S Rule Nitrogen

Hunds rule of maximum multiplicity rule states that for a given electron configuration the term with maximum multiplicity falls lowest in energy.
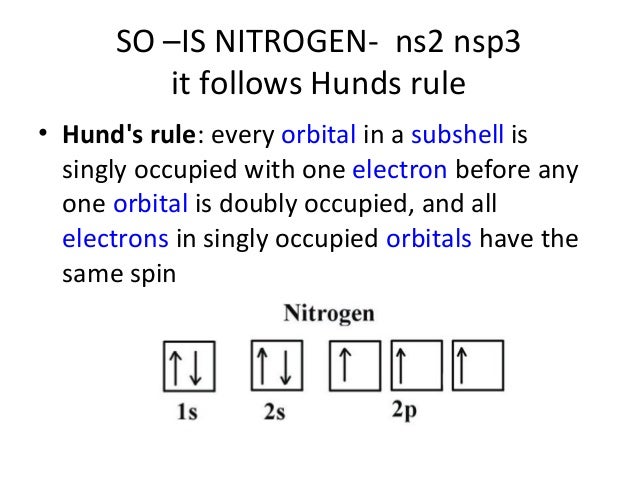
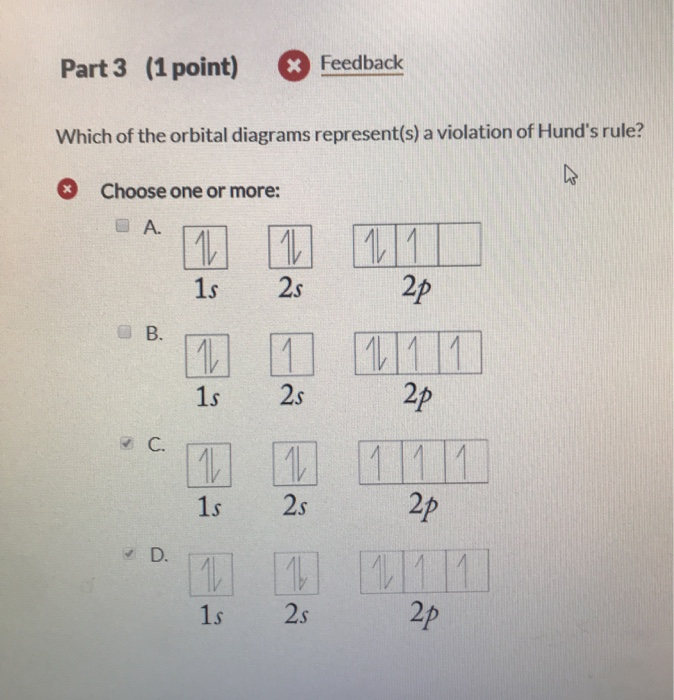
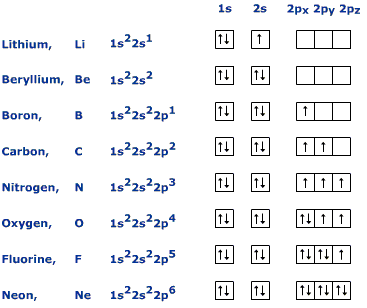
Hund s rule nitrogen. According to this rule electron pairing in p d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied. For a given electron configuration the term.
Electrons tend to minimize repulsion by occupying their own orbitals rather than sharing an orbital with another electron. Orbital filling diagrams an orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.
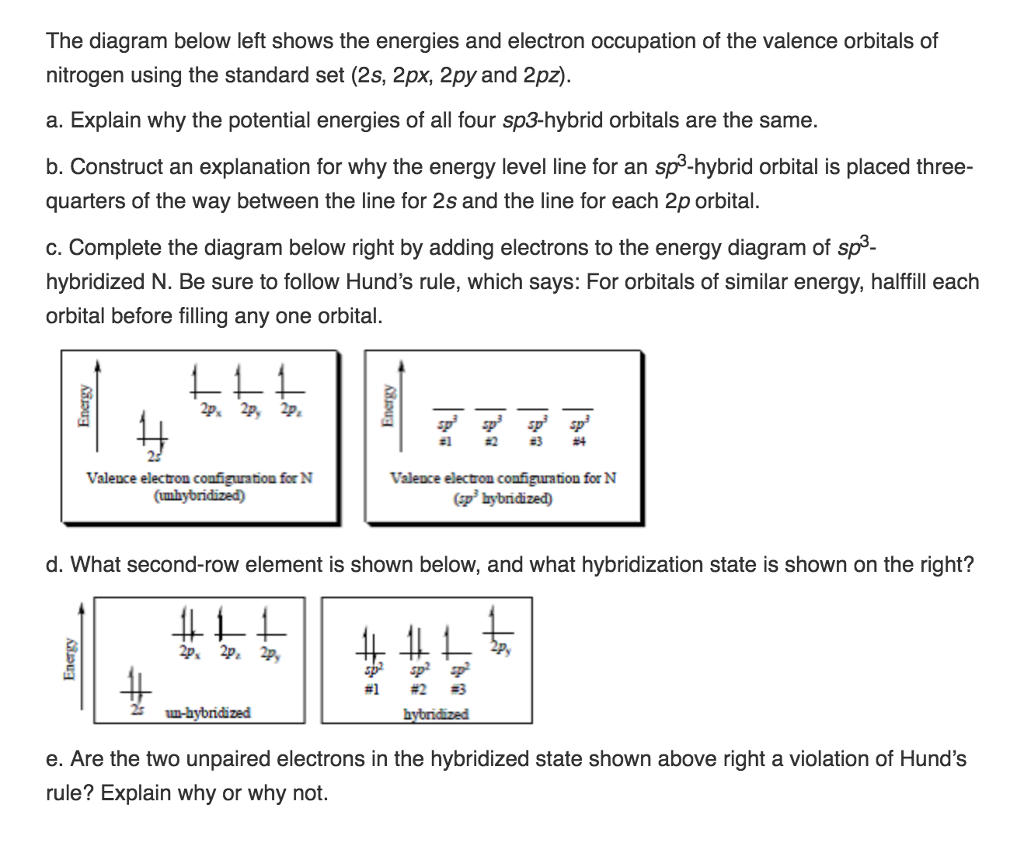
According to the first rule electrons always enter an empty orbital before they pair up. Hund s rules a set of guidelines known as hund s rules help us determine the quantum numbers for the ground states of atoms.
All of the electrons in singly occupied orbitals have a similar spin to maximize total spin. According to hund s rule as electrons are added to a set of orbitals of equal energy one electron enters each orbital before any orbital receives a second electron.
The three rules are. Hund s rule in the electronic configuration filling electrons to orbitals electrons first fill singly to all orbitals with similar energy before pairing with another electron in a half filled orbital.
The hydrogenic shells fill up giving well defined states for the closed shells. Electrons are negatively charged and as a result they repel each other.
Solution for state hund s rule in your own words and show its applica tion in the orbital diagram of the nitrogen atom. Hund s rule states that each orbital in a subshell is only obtained before any orbital is double involved.
As we add valence electrons we follow hund s rules to determine the ground state. In atomic physics hund s rules refers to a set of rules that german physicist friedrich hund formulated around 1927 which are used to determine the term symbol that corresponds to the ground state of a multi electron atom the first rule is especially important in chemistry where it is often referred to simply as hund s rule.
We get a great simplification by treating nearly closed shells as a closed shell plus positively charged.