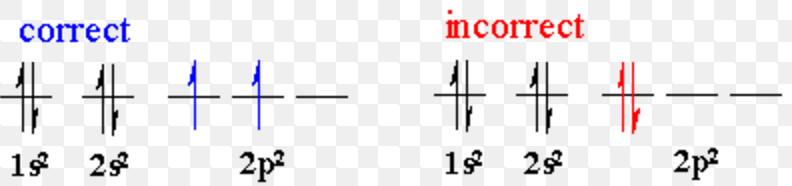Hund S Rule Ncert

Which of the following electronic configuration is not possible according to hund s rule.
Hund s rule ncert. Electrons are small compared to protons and neutrons over 1 800 times less than either a proton or a neutron. All of the electrons in singly occupied orbitals have a similar spin to maximize total spin.
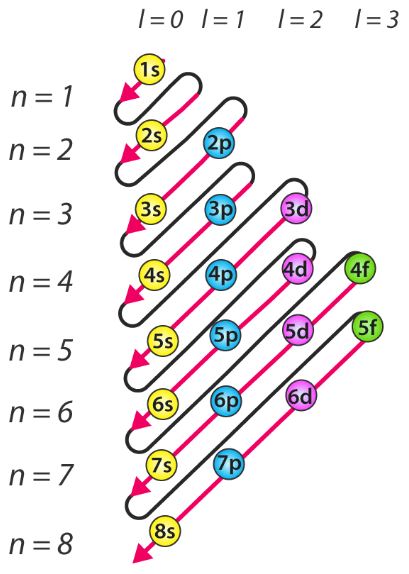
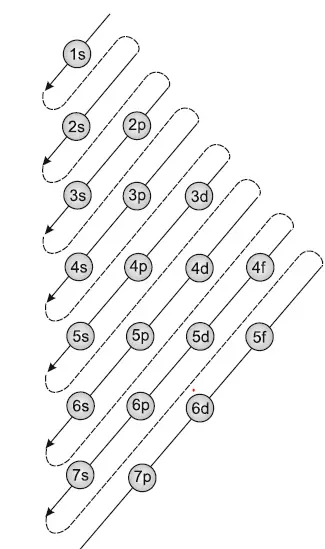
Hund s rule states that each orbital in a subshell is only obtained before any orbital is double involved. Lower n l values correspond to lower.
Every electron should have or be in. Hunds rule electronic configuration pauli s exclusion principle aufbau principle and hunds rule electrons.
Electrons are negatively charged and as a result they repel each other. Class 12 class 11 class 10 class 9 class 8 class 7 class 6.
Electrons have a relative mass of 0 0005439 such that the electron is compared with the mass of a neutron being one or about. Ncert books class 6.
Ncert books class 6. Ncert books for class 5.
Pauli exclusion principle is one of the important principles along with aufbau s principle and hund s rule in chemistry. According to the first rule electrons always enter an empty orbital before they pair up.
Rule where the sum of the principal and azimuthal quantum numbers determines the energy level of the orbital. Ncert books for class 7.
Ncert books for class 7. The manner in which electrons are filled into orbitals in a single subshell must follow hund s rule.
Learning about it is crucial for students especially when they are studying about electrons. Filling of the orbitals goes on according to the aufbau rule aufbau principle.
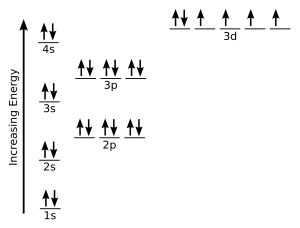
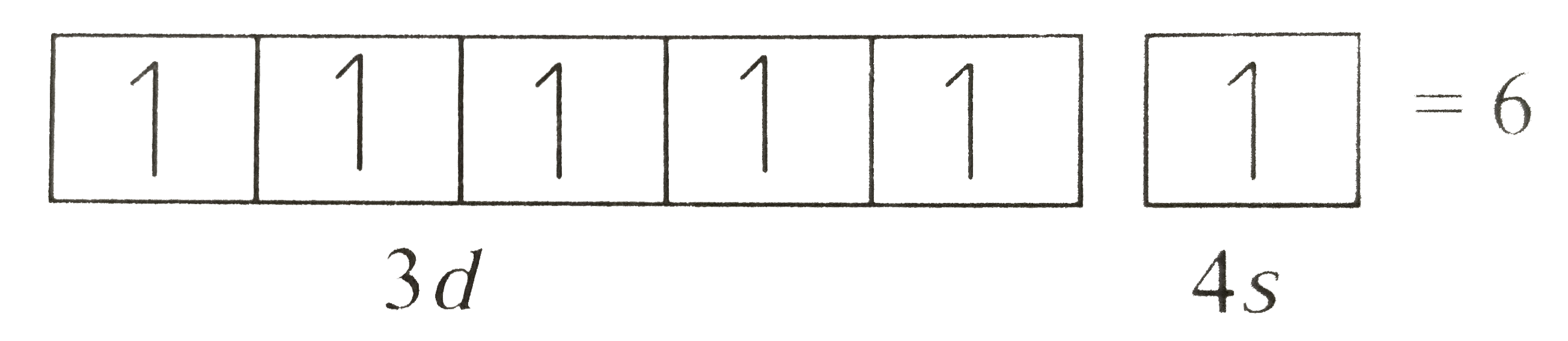
Electrons in the orbitals of given subshell are filled such that each orbital get one electron each with spin in same direction and after that electron pairing occurs. However the location the order of the filling of electrons according to their spin while filling in the degenerate orbitals and the spin of the 2 electrons filled in the same orbital itself are further basically governed by the hund s rule and the pauli s.
Hunds rule of maximum multiplicity rule states that for a given electron configuration the term with maximum multiplicity falls lowest in energy. Cbse 11 chemistry 01 some basic concepts of chemistry 28 topics 29 quizzes 01 01 importance of chemistry.
Ncert dc pandey sunil batra hc verma pradeep errorless. Which of the following electronic configuration is not possible according to hund s rule.
Hunds rule of maximum multiplicity.