Hund S Rule Khan Academy

Fill from the bott.
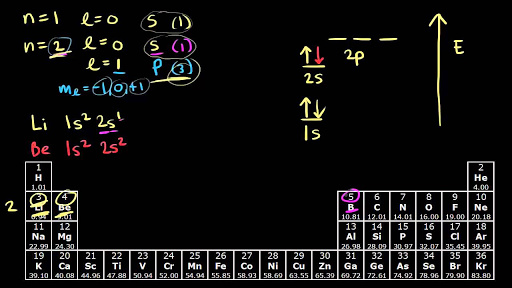
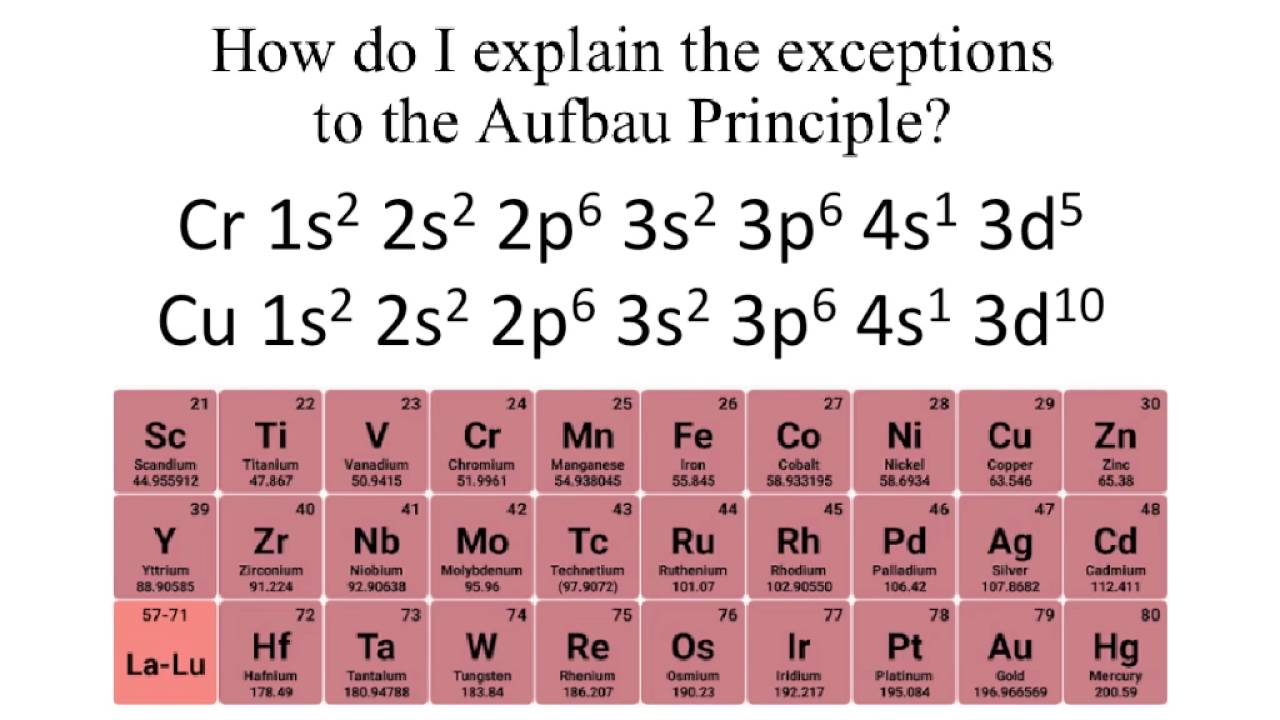
Hund s rule khan academy. Test your knowledge of these. The aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals but this is actually not true for most elements.
Electrons that are not required to have a particular spin by hund s rules can freely change between the and. If you point your pointer finger in the direction the positive charge is moving and then your middle finger in the direction of the magnetic field your thumb points in the direction of the magnetic force pushing on the moving charge.
They are rules we use to fill electron orbital filling diagrams. We can remember this diagram using the right hand rule.
Since monatomic hydrogen has a single electron it is just as likely to be and. From sc on the 3d orbitals are actually lower in energy than the 4s orbital which means that electrons enter the 3d orbitals first.
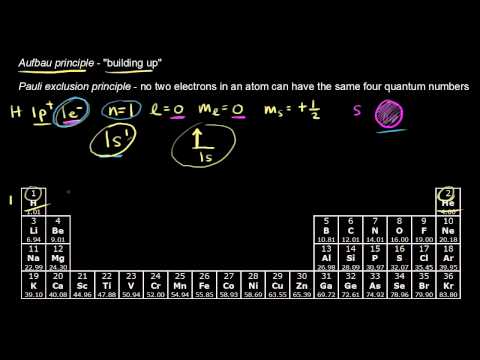
In this video we ll discuss this in more depth and walk through all of the electron configurations for the 3d. 1 electron occupies each orbital and only after all of the orbitals are filled does the orbital get filled with two electrons.
Hund s rule applies to all of the orbitals. If you re seeing this message it means we re having trouble loading external resources on our website.