Hund S Rule Exceptions

Hund s first rule now states that the ground state term is 3 p which has s 1.
Hund s rule exceptions. For heavier elements the j j coupling scheme often gives better agreement with experiment. Learn vocabulary terms and more with flashcards games and other study tools.
The superscript 3 is the value of the multiplicity 2s 1 3. Like most rules there are exceptions.
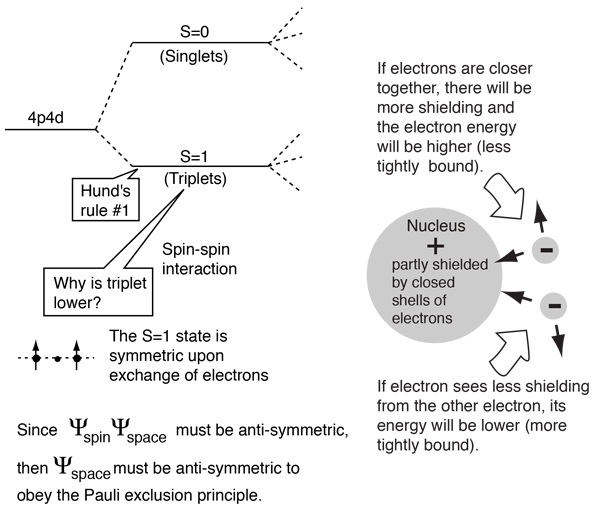
This implies that if two or more orbitals of equal energy are. Electrons are negatively charged and as a result they repel each other.
Exceptions to hund s rules hund s rules presume l s coupling and presume that the electrons can be considered to be in a unique configuration. According to the first rule electrons always enter an empty orbital before they pair up.
Hund s rule states that each orbital in a subshell is only obtained before any orbital is double involved. Aufbau principle exceptions.
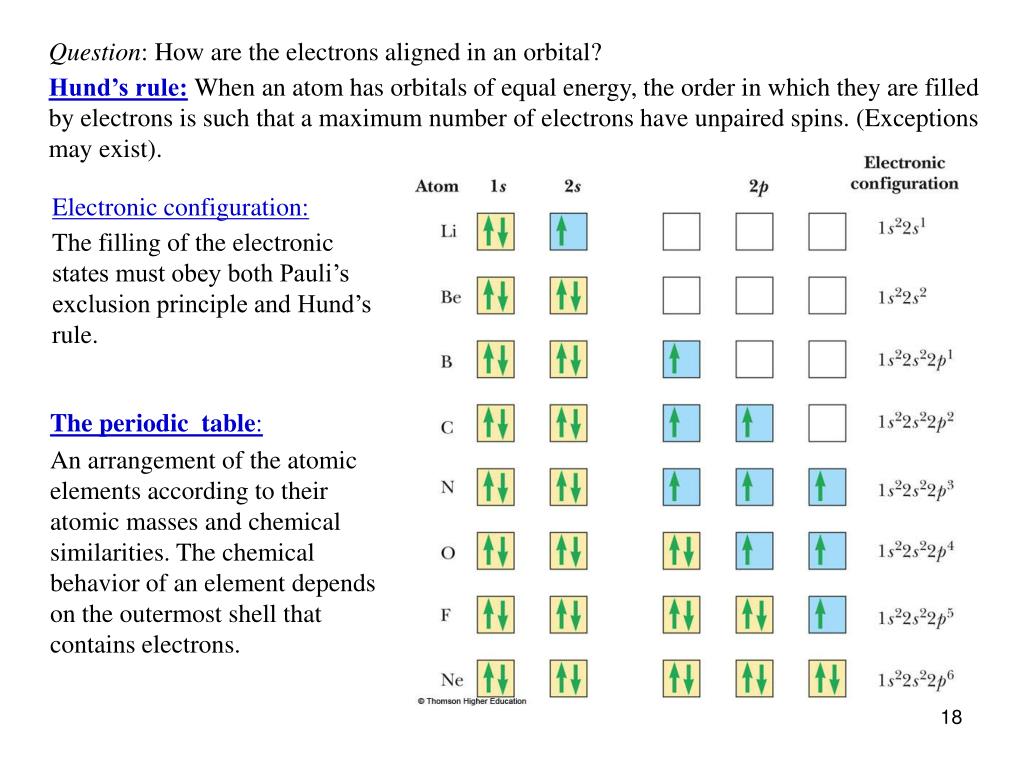
Start studying exceptions to hund s rule. According to this rule electron pairing in p d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied.
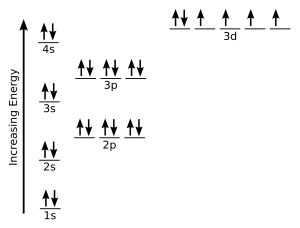
Hund s rule of maximum multiplicity is a rule based on observation of atomic spectra which is used to predict the ground state of an atom or molecule with one or more open electronic shells the rule states that for a given electron configuration the lowest energy term is the one with the greatest value of spin multiplicity. This exception is attributed to several factors such as the increased stability provided by half filled subshells and the relatively low energy gap between the 3d and the 4s subshells.
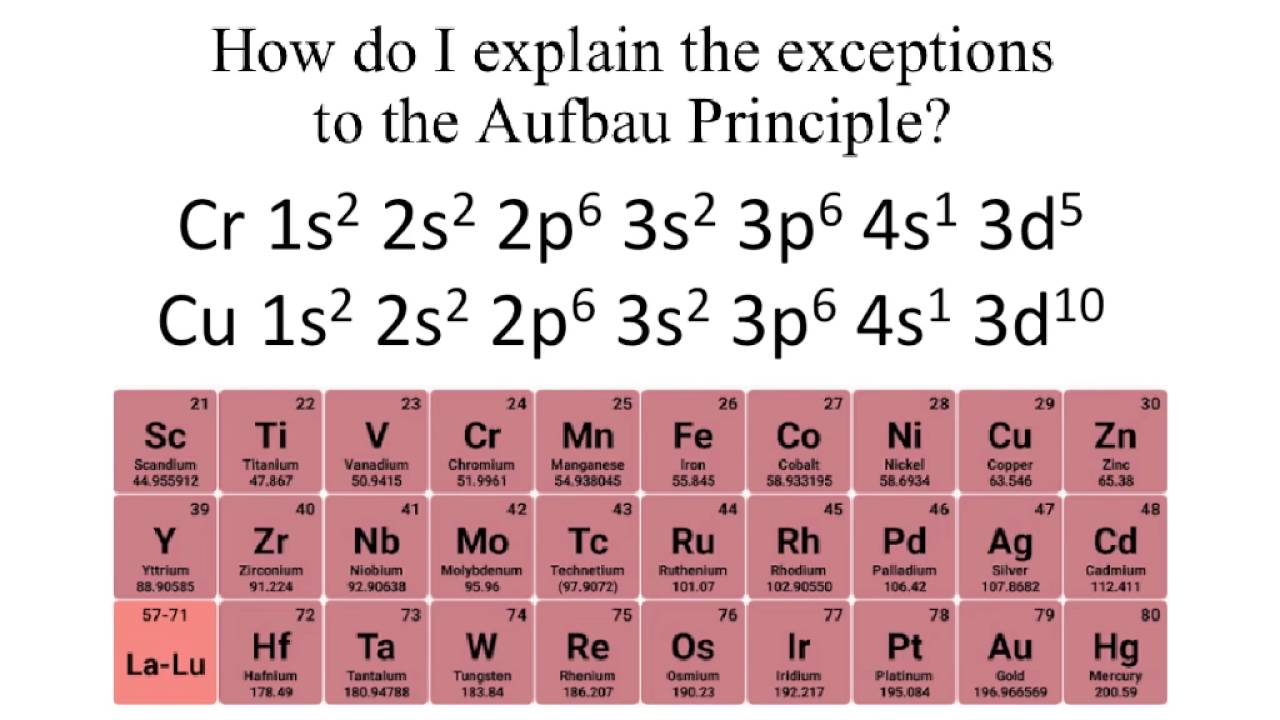
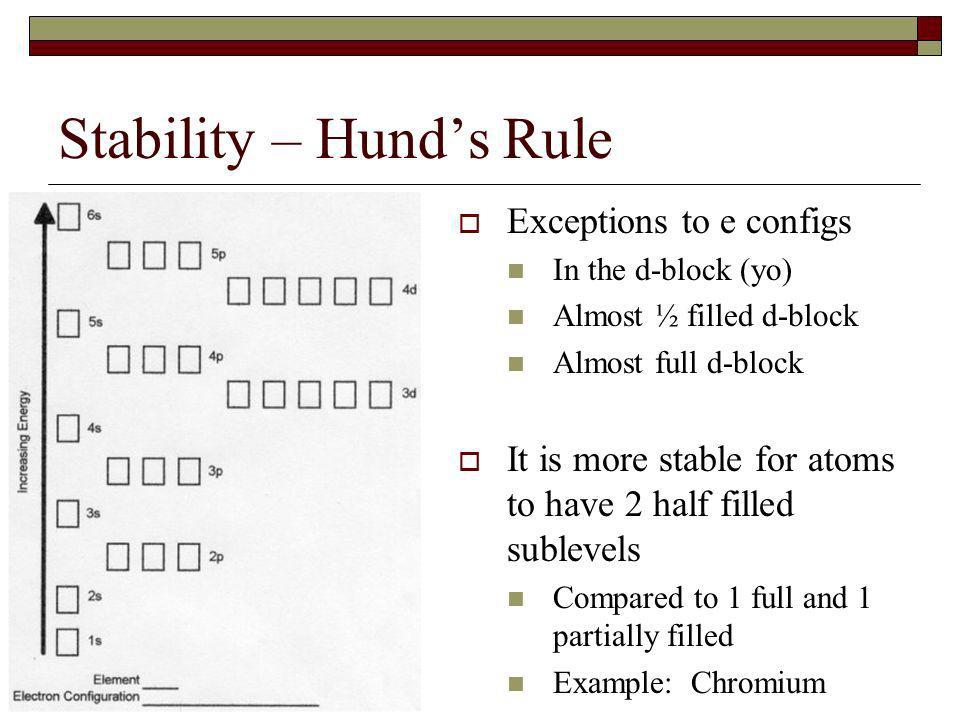
All of the electrons in singly occupied orbitals have a similar spin to maximize total spin. Exceptions the electron configuration of chromium is ar 3d 5 4s 1 and not ar 3d 4 4s 2 as suggested by the aufbau principle.
The diagram shows the state of this term with m l 1 and m s 1. This rule deals with reducing the repulsion between electrons.
For example the predicted aufbau configuration for cr is 4s 2 3d 4 but the observed configuration is actually 4s 1 3d 5. Electrons tend to minimize repulsion by occupying their own orbitals rather than sharing an orbital with another electron.
Half filled and completely filled d and f subshells add stability to atoms so the d and f block elements don t always follow the principle. However it was later found that the atom can be further divided into sub atomic particles after the discovery of the electron by j j.
Hunds rule of maximum multiplicity rule states that for a given electron configuration the term with maximum multiplicity falls lowest in energy. The development of the atomic structure began with dalton s modern atomic theory.