Hund S Law Definition

Hund definition is dialectal variant of hound.
Hund s law definition. This implies that if two or more orbitals of equal energy are available electrons will occupy them singly before filling them in pairs. For a given electron configuration the term with maximum multiplicity has the lowest energy.
The three rules are. Definition of hund s rule hund s rule states that the lowest energy electron configuration the ground state in any electron subshell is the one with the greatest number of parallel electron spins.
The rule discovered by friedrich. Pauli exclusion principle is one of the important principles along with aufbau s principle and hund s rule in chemistry.
Definition what does hund s rule mean. According to hund s rule.
Example 1 consider the different ways in which a pair of electrons might be arranged in p orbitals. All of the electrons in singly occupied orbitals have the same spin to maximize total spin.
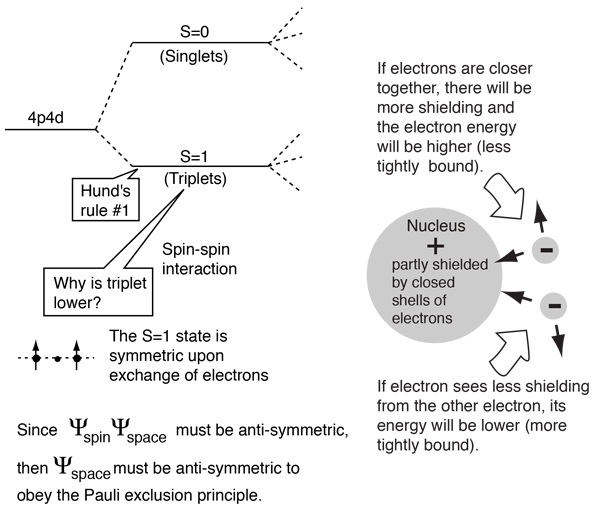
Hund s rule states that a larger total spin state of an atom sometimes makes the atom more stable. The rule states that for a given electron configuration the lowest energy term is the one with the greatest value of spin multiplicity.
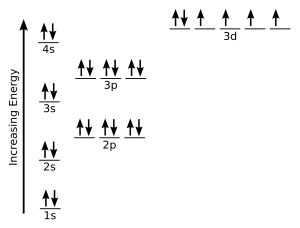
Hund s rule explains the order in which orbitals of subshells are filled electrons. Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
Start your free trial today and get unlimited access to america s largest dictionary with. Each orbital in a sublevel is separately occupied before any orbital is doubly occupied.
More than 250 000 words that aren t in our free dictionary. The multiplicity is equal to 2 s 1 displaystyle 2s.
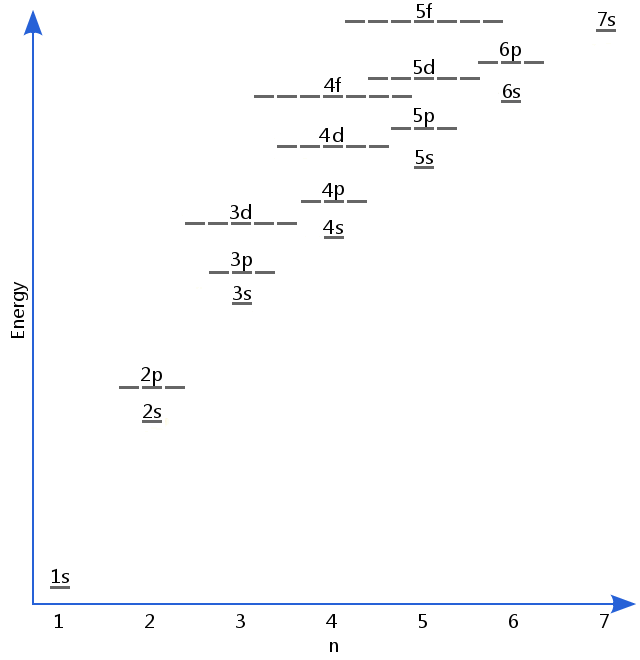
The first rule is especially important in chemistry where it is often referred to simply as hund s rule. Aufbau principle explains the order in which subshells of an atom are filled with electrons.
Learning about it is crucial for students especially when they are studying about electrons. In atomic physics hund s rules refers to a set of rules that german physicist friedrich hund formulated around 1927 which are used to determine the term symbol that corresponds to the ground state of a multi electron atom.
Hund s rule of maximum multiplicity is a rule based on observation of atomic spectra which is used to predict the ground state of an atom or molecule with one or more open electronic shells. You must there are over 200 000 words in our free online dictionary but you are looking for one that s only in the merriam webster unabridged dictionary.
Hunds rule of maximum multiplicity rule states that for a given electron configuration the term with maximum multiplicity falls lowest in energy. Every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied and all electrons in singly occupied orbitals have the same spin.
According to this rule electron pairing in p d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied.