Hund S Law Chemistry In Hindi

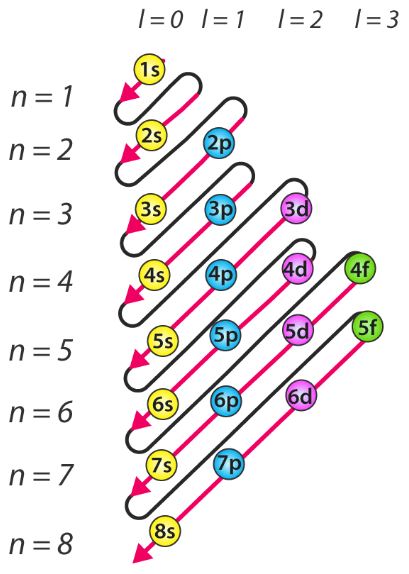
11 according to this rule electron pairing will not take place in orbitals of same energy same sub shell until each orbital is first singly filled with parallel spin.
Hund s law chemistry in hindi. 1s 2 2s 2 2p 2. In this chemistry video in hindi for class 11 we explained pauli exclusion principle which states that no two electrons in an atom can have the same set of f.
Chemistry long question explanation of hund s rule of maximum multiplicity class. According to this rule electron pairing in p d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied.
All of the electrons in singly occupied orbitals have a similar spin to maximize total spin. Hunds rule of maximum multiplicity rule states that for a given electron configuration the term with maximum multiplicity falls lowest in energy.
In other words in a set of orbitals having same energy degenerate orbitals the electrons distribute themselves to occupy separate orbitals. The three rules are.
Hund s rule of maximum multiplicity is a rule based on observation of atomic spectra which is used to predict the ground state of an atom or molecule with one or more open electronic shells the rule states that for a given electron configuration the lowest energy term is the one with the greatest value of spin multiplicity. Hund s law in hindi ह ण ड क न यम प रथम द व त य न यम य अध कतम बह कत क न यम स प क ट र र स यन क श र ण.
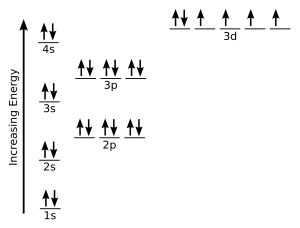
This implies that if two or more orbitals of equal energy are. The two 2s electrons will occupy the same orbital whereas the two 2p electrons will be in different orbital and aligned the same direction in accordance with hund s rule.
Consider the electron configuration for carbon atoms. Definition of hund s rule.
Consider also the electron configuration of oxygen. Hund s rule states that each orbital in a subshell is only obtained before any orbital is double involved.
For a given electron configuration the term.